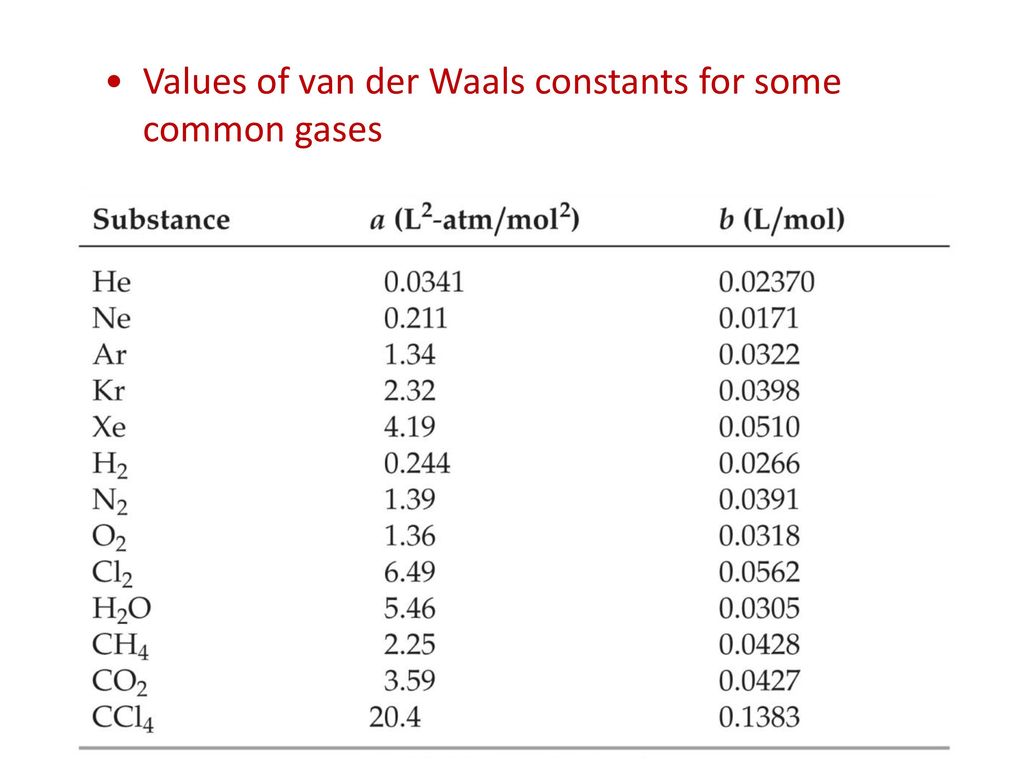
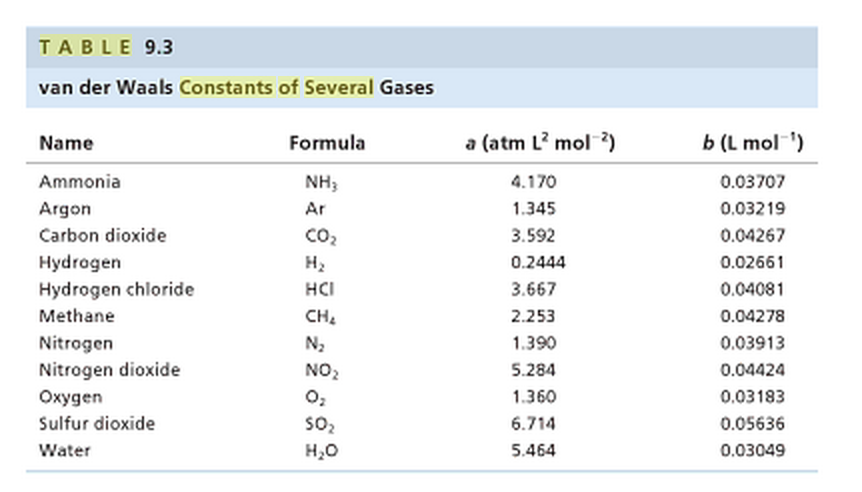
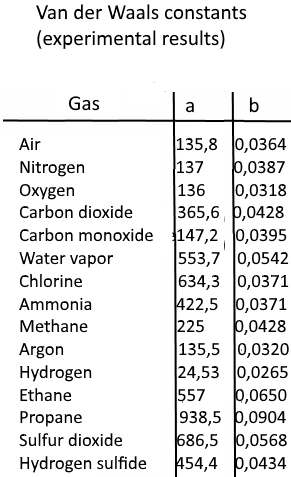
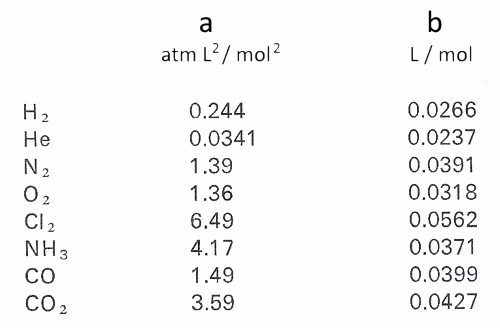
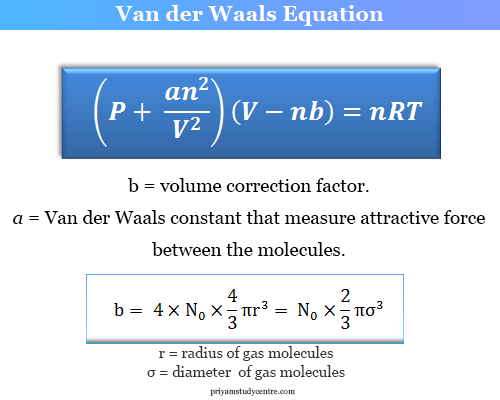
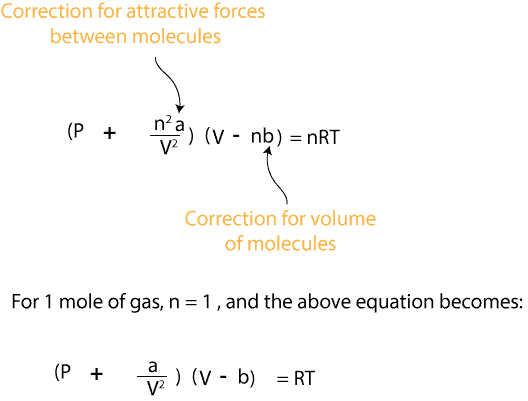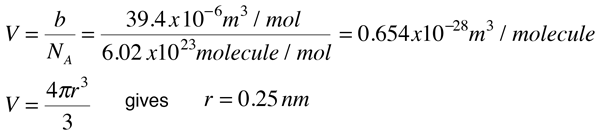For real gas van der Waals equation is written as: ( p + an^2V^2 ) ( V - nb ) = nRT Where a and b are van der Waals constants.Two sets

OneClass: Based on the van der Waals equation of state and given the van der Waals constants below, w...

11111 Umu) 32 min 46. The ratio of van der Waals' constants a and b, has the dimension of lá atm L- ((b) L atm mol-' (c) L mol-1 (d) atm L
Which of the following expressions represent the value and unit of van der Waals' constant a? - Sarthaks eConnect | Largest Online Education Community

Which of the following statements regarding van der Waals' constants a and b is not correct ? (a) The constant a is a measure of van der Waals' forces (b) A gas
What are the symbols 'R' (ideal gas constant), 'a' and 'b' (Van der Waals constants) abbreviations for? - Quora
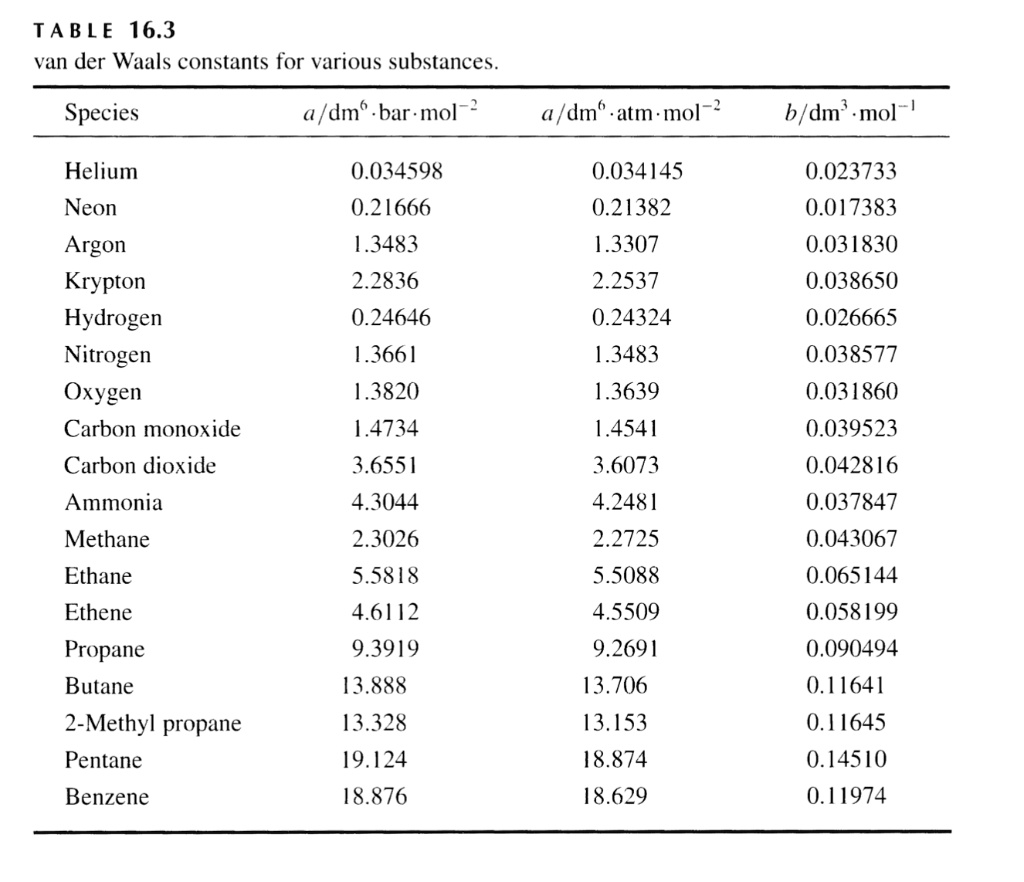
SOLVED:TA BL E 16.3 van der Waals constants for various substances Species /dm6 . bar-mol dm" . atm- mol b/dm'. mol Helium Neon Argon Krypton Hydrogen Nitrogen Oxygen Carbon monoxide Carbon dioxide

physical chemistry - Why does small value of van der Waals gas constant "b" ensure easier liquefication? - Chemistry Stack Exchange

The ratio of van der Waals' constants a and b, has the dimension of (a) atm ? (C) L mol-1 (b) L atm mol-1 (d) atm L mol-2

At T=300K, 1.00mol of CO2 occupies a volume of 1.50L. Calculate the pressures given by the ideal gas equation and the van der Waals equation. (The van der Waals constants a and

Consider the Vander Waals constant , a and b , for the following gases Which gas is expected to have the highest critical temperature - Sahay LMS

homework and exercises - Van der Waals constant $b$ (real gas) chemical form. only - Physics Stack Exchange